Does Sodium Have 2 Valence Electrons
We know that C has 4 valence electrons and that O has 6 valence electrons which means that the number of. 2 electrons in the first shell 8 electrons in the second shell.
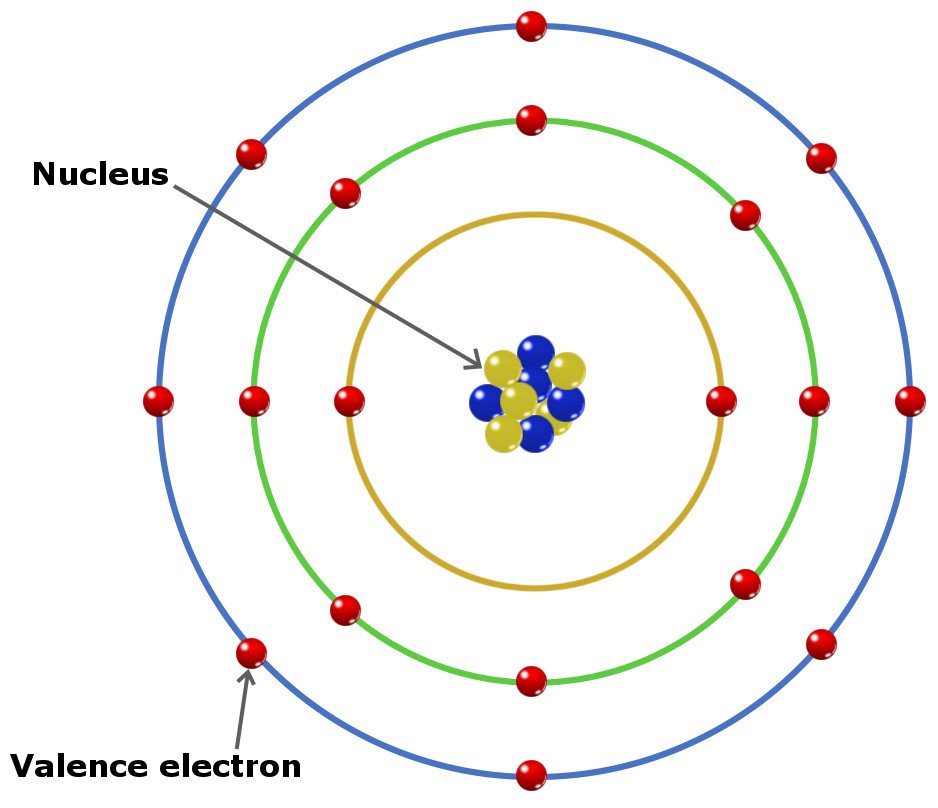
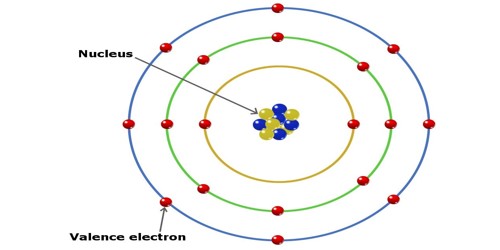
What Are Valence Electrons And How To Find Them Where Are They Located
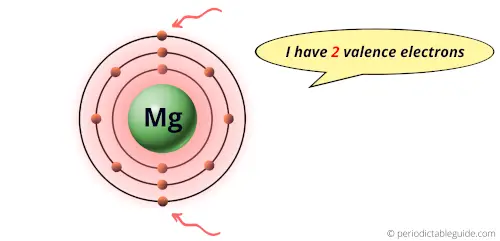
Two valence electrons are present in Group 2s element.
. The third shell is a bit more complicated but lets just say that it takes up to 8 electrons as well for now. The elements that form bonds by donating electrons are called cations. The tendency is for sodium to lose an electron so that the new resulting valence shell 2 is in its most stable state full octet.
Each carbon dioxide molecule is formed from 1 C atom and 2 O atoms. How many electrons does sodium fluoride have. So a fluoride ion F has 10 electrons rather than 9 why a neutral neon atom has 10 electrons and a sodium ion Na also has 10 electrons why.
The second shell can have up to 8 electrons. Therefore a sodium atom will have two electrons in the first shell eight in the 2nd orbit and an electron in the 3rd shell. And 1 electron the valence electron in the.
Its atomic number is 11 so it has 11 protons. The elements that have 1 2 or 3 electrons in the last shell donate the electrons in the last shell during bond formation. That gives a total of 7 electrons so neutral chlorine atoms have 7 valence electrons.
How Many Electrons Does Sodium Need To Gain To Become Stable. Atoms are neutral so this means that sodium also has 11 electrons Electrons are arranged in shells or energy levels. The elements that form bonds by donating electrons are called cation.
Does sodium tend lose or gain electrons and how many. How many valence electrons does chromium ionCr 2Cr 3 have. Therefore the order of the number of electrons in each shell of the sodium atom is 2 8 1.
It is now known as a sodium ion. Therefore valence electron in sodium is 1 and it needs to lose 1 electron from the outermost orbit to attain octet. The first evel can have 2 electrons.
Sodium has an atomic number of 11 and so therefore in a neutral state one atom of sodium has 11 protons and 11 electrons. The atomic number of sodium Na is 11. The number of valence electrons for molecules can be calculated by adding the valence electrons of all the atoms that form that respective molecule.
Hence the valency of sodium is 1. The third shell is a bit more complicated but lets just say that it takes up to 8 electrons as well for now. Does sulfur and oxygen have 6 valence electrons.
The elements that have 1 2 or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The sulfur atom shares four valence electrons with two oxygen atoms as two covalent double bonds and shares its remaining two electrons with two oxygen atoms forming two covalent single bonds. Valency of Sodium The electronic configuration of sodium can be written as 2 8 1.
And 1 electron the valence electron in the third shell. The last number is how we know the number of valence electrons. Strontium atom donates two electrons of the last shell.
How many valence electrons does sodium ionNa have. Now Z refers to the number of protons in the elements nucleus and protons are POSITIVELY charged particles. Na is a sodium atom that has lost an electron to form a positive ion.
Atoms like sodium with only one or two electrons in a valence shell that needs eight electrons are most likely to give up their valence electrons to achieve a stable state. This loss of an electron results in the ionization of sodium to form the positively charged ion Na. That is the number of electrons in sodium is 11.
2 8 1 electrons are distributed in the shells K L M respectively. The second shell can have up to 8 electrons. The highest-numbered shell is the third shell which has 2 electrons in the 3s subshell and 5 electrons in the 3p subshell.
Here are some examples. 2 electrons in the first shell 8 electrons in the second shell. And 1 electron the valence electron in the.
So sodiums 11 electrons are arranged this way. To fill the outer shell sodium takes less energy than it does to accept seven more electrons. Valency and valence electrons of bromine This electron configuration shows that the last shell of a bromine atom has an unpaired electron.
In summary Sulfur is neutral in SO4 and has six valence electrons. After the electron configuration the last shell of the sodium atom has an electron. So sodiums 11 electrons are arranged this way.
Why does sodium have 10 electrons. If sodium loses an electron it now has 11 protons 11 neutrons and just 10 electrons leaving it with a 1 charge. Valence electrons are the outermost electrons and are the ones involved in bonding.
2 electrons in the first shell 8 electrons in the second shell. So sodiums 11 electrons are arranged this way. The correct electron configuration of bromineBr in the ground state will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p x 2 4p y 2 4p z 1.
Sodium has 11 electrons. We know the details about this. 1 how many valence electrons and how many core electrons does a neutral chlorine atom have.
We write this as 281. The second level can have up to 8. An ion is any atom that has gained electrons to have a negative charge anion.
In this case the valency of sodium is 1. The third shell is a bit more complicated but lets just say that it takes up to 8 electrons as well for now. The elements that have 1 2 or 3 electrons in the last shell donate the electrons in the last shell during bond formation.

Science Coverage Valency Of Lithium How Many Valence Electrons Do Electron Configuration Electrons Chemistry

How To Find Valency What Are Valence Electrons Teachoo
Periodic Table With Valence Electrons Labeled 7 Hd Images

How Many Valence Electrons Does Selenium Se Have

Valence Electrons Assignment Point

How Many Valence Electrons Does Chlorine Cl Have
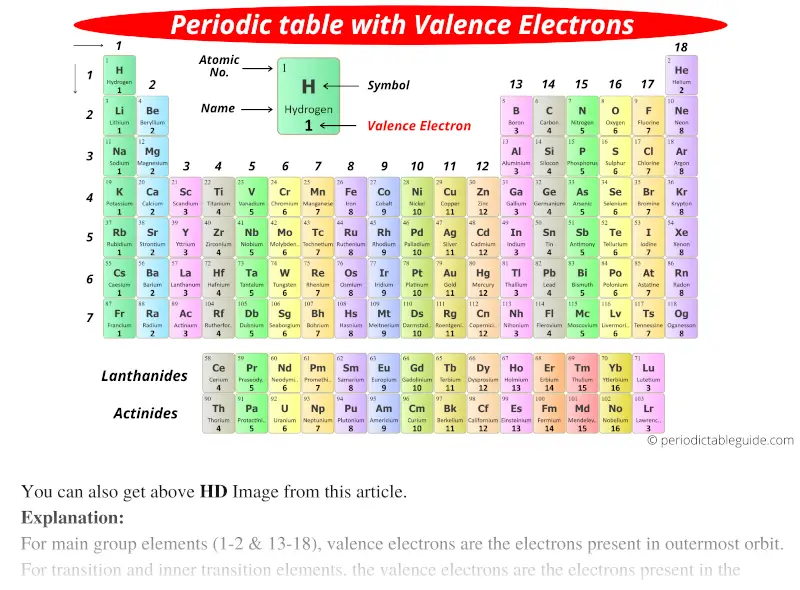
Periodic Table With Valence Electrons Labeled 7 Hd Images

How Many Valence Electrons Does Titanium Ti Have
How Many Valence Electrons Does S Have Quora
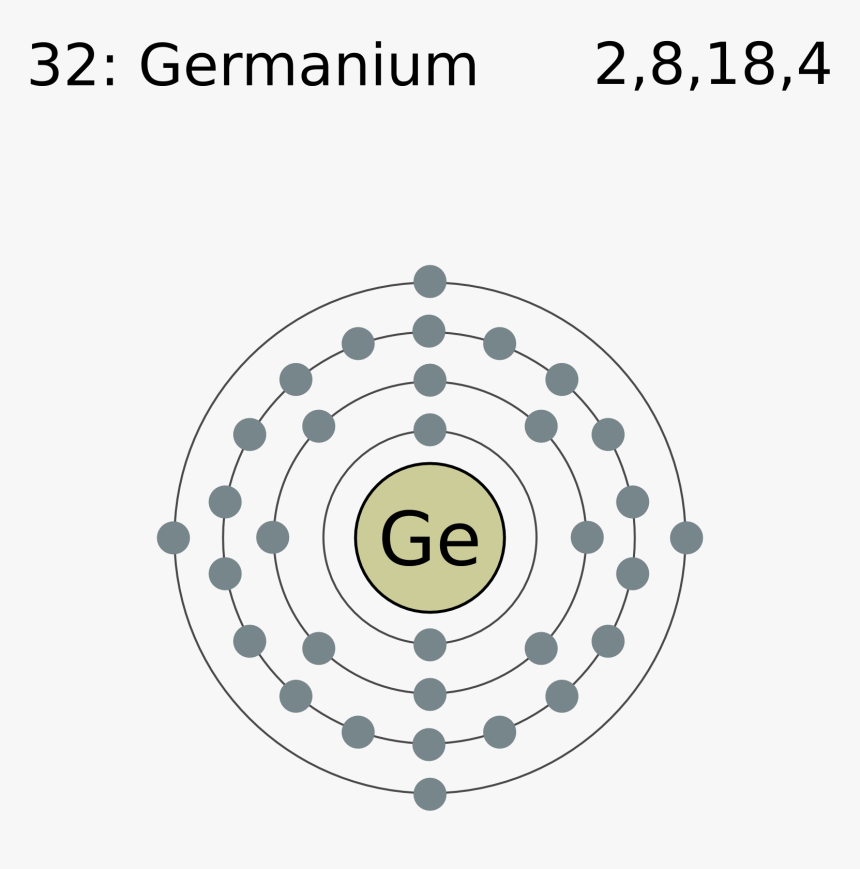
Electron Shell 032 Germanium Germanium Valence Electrons Hd Png Download Kindpng

How Many Valence Electrons Does Niobium Nb Have

How Many Valence Electrons Does Chromium Cr Have

Number Of Valence Electrons For Sodium Na Youtube

Valency And Valence Electrons Definition Examples And Relation Geeksforgeeks
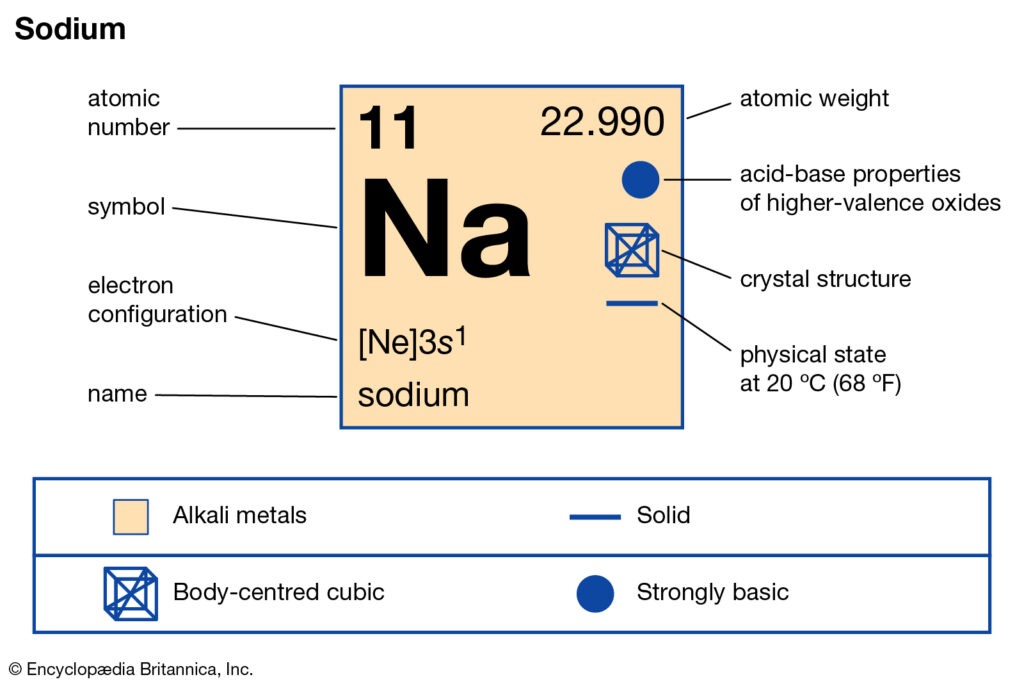
Sodium Valence Electrons Sodium Valency Na With Dot Diagram

How Many Valence Electrons Does Sodium Na Have


Comments
Post a Comment